Primary extraskeletal osteosarcoma of the omentum in a young dog
DOI:
https://doi.org/10.24070/bjvp.1983-0246.018018Keywords:
canine osteosarcoma, histopathology, immunohistochemistry, metastasis, RUNX2Abstract
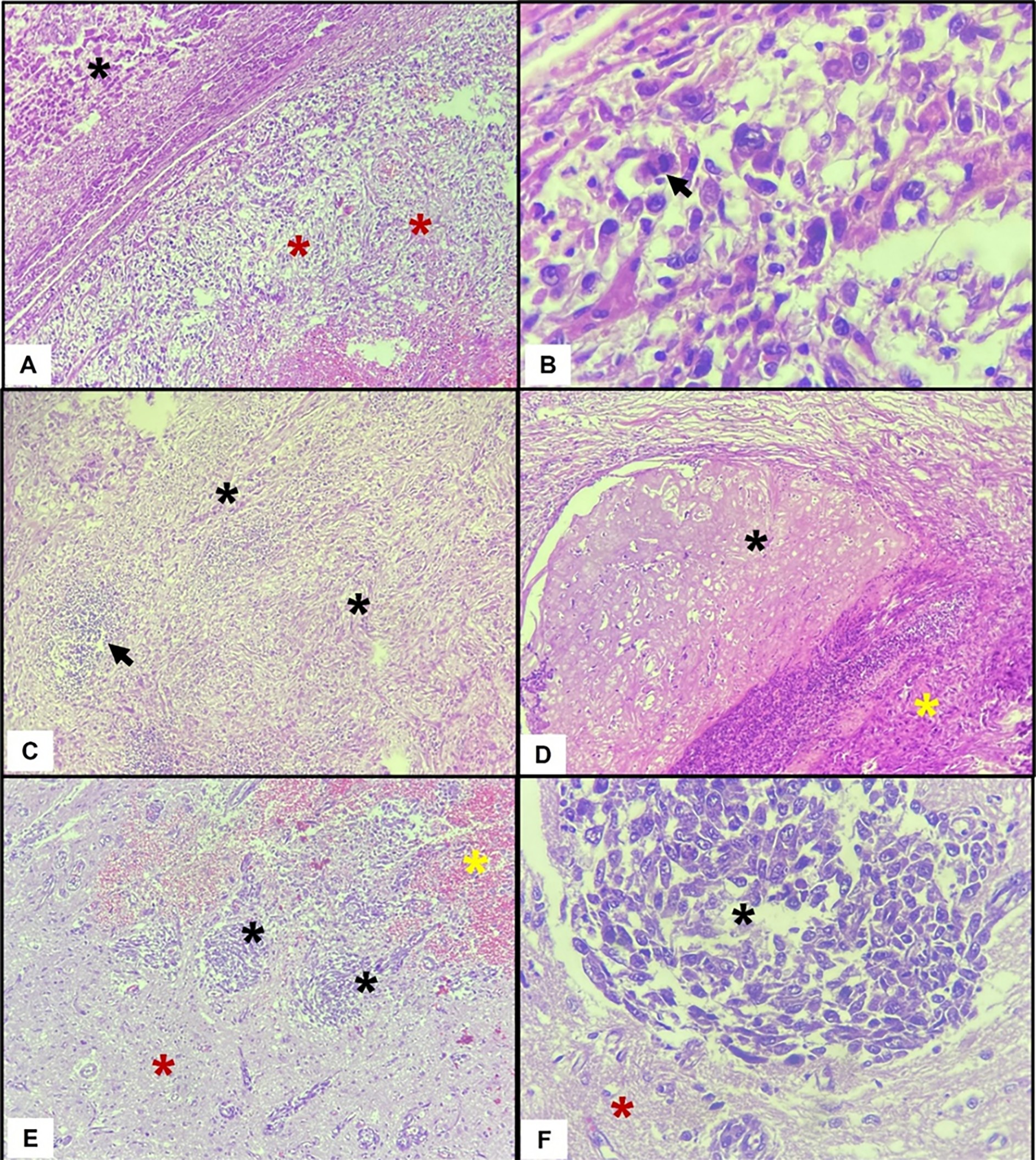
Extraskeletal osteosarcoma (EOSA) is a rare and aggressive mesenchymal neoplasm in dogs, primarily affecting older animals, with the spleen being the most common site of involvement. This report describes a rare case of primary EOSA originating in the omentum of a 1-year-and-5-month-old spayed female dog, highlighting the role of immunohistochemistry, specifically the positive expression of vimentin and RUNX2, in confirming the tumor’s mesenchymal and osteoblastic origin. The tumor was inoperable, and treatment consisted of medical management with toceranib phosphate and losartan, with the addition of zoledronic acid following the detection of bone metastasis. The patient exhibited temporary clinical improvement for 90 days, after which disease progression was noted, including the development of abdominal effusion (1.2 liters drained). The patient survived for 180 days following diagnosis, without adverse effects related to the treatment. Necropsy confirmed widespread metastases from fibroblastic osteosarcoma, including the skin, peritoneum, lungs, heart, liver, lymph nodes, and brain, with cerebral hemorrhage as the likely cause of sudden death. Histopathological evaluation revealed extensive areas of osteoid matrix and proliferating mesenchymal cells in a disorganized arrangement, with marked vascular invasion by neoplastic emboli, as well as multifocal hemorrhage and tumor necrosis. This case illustrates a rare presentation of EOSA in a young dog and emphasizes the importance of including neoplasia in the differential diagnosis of abdominal masses, regardless of patient age. It also highlights the diagnostic value of immunohistochemical markers, particularly RUNX2, in distinguishing EOSA from other mesenchymal or reactive proliferations.
References
Amaral CB, Leite J da S, Fonseca ABM, Ferreira AMR. Vimentin, osteocalcin and osteonectin expression in canine primary bone tumors: diagnostic and prognostic implications. Mol Biol Rep. 2018;45(5):1289-96. doi: 10.1007/s11033-018-4285-6.
Barger A, Baker K, Driskell E, Sander W, Roady P, Berry M, et al. The use of alkaline phosphatase and runx2 to distinguish osteosarcoma from other common malignant primary bone tumors in dogs. Vet Pathol. 2022;59(3):427-32. doi: 10.1177/03009858221083035.
Chang J, Wang W, Zhang H, Hu Y, Yin Z. Bisphosphonates regulate cell proliferation, apoptosis and pro-osteoclastic expression in MG-63 human osteosarcoma cells. Oncol Lett. 2012;4(2):299-304. doi: 10.3892/ol.2012.723.
Conry RM, Rodriguez MG, Pressey JG. Zoledronic acid in metastatic osteosarcoma: encouraging progression free survival in four consecutive patients. Clin Sarcoma Res. 2016;6(1):6. doi: 10.1186/s13569-016-0046-2.
Cristo T, Vargas C, Biezus G, Costa L, Pont T, Kanamura C, et al. Metastatic osteosarcoma as a cause of hemorrhagic stroke in a dog. Braz J Vet Pathol. 2017;10(3):105-10. doi: 10.24070/bjvp.1983-0246.v10i3p105-110.
Duffy D, Selmic LE, Kendall AR, Powers BE. Outcome following treatment of soft tissue and visceral extraskeletal osteosarcoma in 33 dogs: 2008–2013. Vet Comp Oncol. 2017;15(1):46–54. doi: 10.1111/vco.12141.
Fontana C, Novaski E, Montagnini K, Lima S, Druziani J, dos Santos E, et al. Sclerosing encapsulating peritonitis in a dog. Braz J Vet Pathol. 2022;15(3):133-8. doi: 10.24070/bjvp.1983-0246.v15i3p133-138.
Giuliano A, Horta RS, Vieira RAM, Hume KR, Dobson J. Repurposing drugs in small animal oncology. Animals. 2022;13(1):139. doi: 10.3390/ani13010139.
Holanda AGA, Marangoni LA, Dos Anjos DS. Long-term survival in a dog with unresectable appendicular osteosarcoma treated with zoledronic acid plus carboplatin as palliative care. Acta Vet Bras. 2025;19(1):14-8. doi: 10.21708/avb.2025.19.1.12635.
Kim C, Matsuyama A, Mutsaers AJ, Woods JP. Retrospective evaluation of toceranib (Palladia) treatment for canine metastatic appendicular osteosarcoma. Can Vet J. 2017;58(10):1059-64.
Kuntz C, Dernell W, Powers B, Withrow S. Extraskeletal osteosarcomas in dogs: 14 cases. J Am Anim Hosp Assoc. 1998;34(1):26-30. doi: 10.5326/15473317-34-1-26.
Langenbach A, Anderson M, Dambach D, Sorenmo K, Shofer F. Extraskeletal osteosarcomas in dogs: a retrospective study of 169 cases (1986-1996). J Am Anim Hosp Assoc. 1998;34(2):113-20. doi: 10.5326/15473317-34-2-113.
Leonardi L, Manuali E, Bufalari A, Porcellato I. Canine soft tissue sarcomas: the expression of RUNX2 and karyopherin alpha-2 in extraskeletal (soft tissues) and skeletal osteosarcomas. Front Vet Sci. 2024;11:1292852. doi: 10.3389/fvets.2024.1292852.
Ouyang Z, Li H, Zhai Z, Xu J, Dass CR, Qin A, et al. Zoledronic acid: pleiotropic anti-tumor mechanism and therapeutic outlook for osteosarcoma. Curr Drug Targets. 2018;19(5):409-21. doi: 10.2174/1573399811666150615145409.
Patnaik AK. Canine extraskeletal osteosarcoma and chondrosarcoma: a clinicopathologic study of 14 cases. Vet Pathol. 1990;27(1):46-55. doi: 10.1177/030098589002700107.
Pazzi P, Tompkins S, Kirberger RM. Canine spirocercosis-associated extraskeletal osteosarcoma with central nervous system metastasis. J S Afr Vet Assoc. 2013;84(1):E1-4. doi: 10.4102/jsava.v84i1.71.
Regan DP, Chow L, Das S, Haines L, Palmer E, Kurihara JN, et al. Losartan blocks osteosarcoma-elicited monocyte recruitment, and combined with the kinase inhibitor toceranib, exerts significant clinical benefit in canine metastatic osteosarcoma. Clin Cancer Res. 2022;28(4):662-76. doi: 10.1158/1078-0432.CCR-21-2105.
Smith AA, Lindley SES, Almond GT, Bergman NS, Matz BM, Smith AN. Evaluation of zoledronate for the treatment of canine stage III osteosarcoma: A phase II study. Vet Med Sci. 2023;9(1):59-67. doi: 10.1002/vms3.1000.
Spugnini EP, Vincenzi B, Caruso G, Baldi A, Citro G, Santini D, et al. Zoledronic acid for the treatment of appendicular osteosarcoma in a dog. J Small Anim Pract. 2009;50(1):44-6. doi: 10.1111/j.1748-5827.2008.00635.x.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Brazilian Journal of Veterinary Pathology

This work is licensed under a Creative Commons Attribution 4.0 International License.

