Clinicopathologic features, tumor staging, and intratumoral inflammation in canine oral non-tonsillar squamous cell carcinomas
DOI:
https://doi.org/10.24070/bjvp.1983-0246.018019Keywords:
oncology, cancer, tumor infiltration, neutrophils, dogAbstract
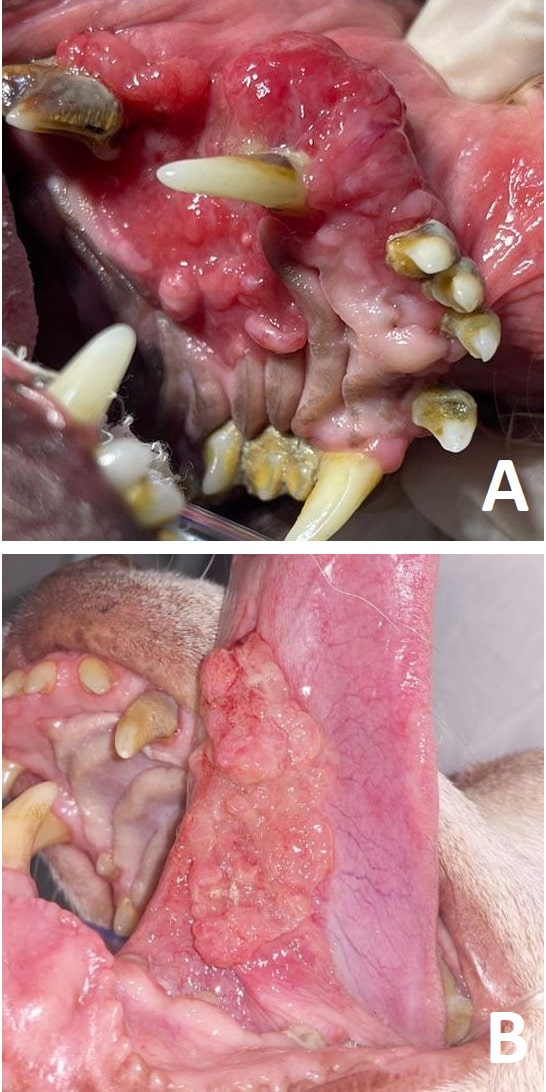
Oral cancers, such as squamous cell carcinomas, constitute important diseases in veterinary patients, as they are frequently observed in pets. Their usually aggressive behavior makes it critical to characterize the clinicopathologic features that can provide useful data for decision-making. Recently, the importance of inflammatory cell infiltration in different tumor types has been highlighted due to its association with clinical findings and outcomes. Thus, this study aimed to evaluate clinicopathologic features in 62 cases of canine oral non-tonsillar squamous cell carcinomas, as well as to investigate tumor infiltration by inflammatory cells and its relation to clinical staging. Statistically significant results were observed when comparing neutrophil infiltration in stage 2 patients and animals presenting higher stages (3 and 4). Patients in stages 3 and 4 revealed lower neutrophil infiltration than stage 2 dogs. Histological grading showed differences in mitotic count between less differentiated and well/moderately differentiated neoplasms. Such features—especially those related to neutrophil infiltration and tumor staging—are relevant for the comprehension of non-tonsillar tumors, consequently aiding in decision-making in cases of canine oral squamous cell carcinoma in dogs.
References
Balkwill F. The chemokine system and cancer. J Pathol. 2012;226(2):148–57.
Boss MK, Harrison LG, Gold A, Karam SD, Regan DP. Canine oral squamous cell carcinoma as a spontaneous, translational model for radiation and immunology research. Frontiers in Oncology. 2023;12:1033704. doi:10.3389/fonc.2022.1033704.
Bryne MM, Koppang HS, Lilleng R, Kjaerheim A. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol. 1992;166:375–81.
Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16(7):431–46.
Cray C, Selmic LE, Ruple A. Demographics of dogs and cats with oral tumors presenting to teaching hospitals: 1996–2017. J Vet Sci. 2020;21:e70.
Dobson JM, Samuel S, Milstein H, Rogers K, Wood JLN. Canine neoplasia in the UK: estimates of incidence rates from a population of insured dogs. J Small Anim Pract. 2002;43:240–6.
Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest. 2014;124(12):5466–5480. doi:10.1172/JCI77053.
Fulton A, Nemec A, Murphy BG, Kass PH, Verstraete FJM. Risk factors associated with survival in dogs with non-tonsillar oral squamous cell carcinoma: 31 cases (1990–2010). J Am Vet Med Assoc. 2013;242:696–702.
Granot Z, et al. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2017;32(3):300–14.
Gu FM, et al. Intratumoral IL-17 cells and neutrophils show strong prognostic significance in intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2012;19:2506–14.
Hanahan D, Weinberg R. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46.
Hu X, et al. Neutrophils promote tumor progression in oral squamous cell carcinoma by regulating EMT and JAK2/STAT3 signaling through chemerin. Front Oncol. 2022;12:812044.
Ilie M, et al. Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer. 2012;118:1726–37.
Iwai N, Okuda T, Sakagami J. Neutrophil to lymphocyte ratio predicts prognosis in unresectable pancreatic cancer. Sci Rep. 2020;10:18758.
Lang NP, Bartold PM. Periodontal health. J Periodontol. 2018;89:S9–16.
Li YW, et al. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011;54:497–505.
Liu D, et al. Canine spontaneous head and neck squamous cell carcinomas represent their human counterparts at the molecular level. PLoS Genet. 2015;11(6):e1005277.
Ma X, et al. Definition of prostaglandin E2-EP2 signals in the colon tumor microenvironment that amplify inflammation and tumor growth. Cancer Res. 2015;75:2822–33.
Masucci MT, Minopoli M, Carriero MV. Tumor-associated neutrophils: their role in tumorigenesis, metastasis, prognosis, and therapy. Front Oncol. 2019;9:1146.
McAllister SS, Weinberg R. The tumor-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol. 2014;16:717–27.
Mestrinho L, et al. Comparison of histological and proliferation features of canine oral squamous cell carcinoma based on intraoral location: 36 cases. J Vet Dent. 2017;34:92–7.
Mestrinho L. Current status and future perspectives in canine oral squamous cell carcinoma. Vet Pathol. 2018;55:200–1.
Nagamine E, et al. Invasive front grading and epithelial-mesenchymal transition in canine oral and cutaneous squamous cell carcinomas. Vet Pathol. 2017;54:783–91.
Nemec A, et al. Histological subtypes of oral nontonsillar squamous cell carcinoma in dogs. J Comp Pathol. 2012;147:111–20.
Owen LN. TNM classification of tumours in domestic animals. Geneva: WHO; 1980.
Powell DR, Huttenlocher A. Neutrophils in the tumor microenvironment. Trends Immunol. 2017;38(1):41–52.
Pylaeva E, Lang S, Jablonska J. The essential role of type I interferons in differentiation and activation of tumor-associated neutrophils. Front Immunol. 2016;7:629.
Rao HL, et al. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients' adverse prognosis. PLoS One. 2012;7:e30806.
Ravanbakhsh A, et al. Assessment of neutrophil function in canine cancer patients undergoing chemotherapy and correlation with neutrophil numbers. Can J Vet Res. 2021;85:137–44.
Shaul ME, Fridlender ZG. Tumor-associated neutrophils in patients with cancer. Nat Rev Clin Oncol. 2019;16:601–20.
Shen M, et al. Tumor-associated neutrophils as new prognostic factor in cancer: a systematic review and meta-analysis. PLoS One. 2014;9:e98259.
Shinada M, et al. Podoplanin promotes cell proliferation, survival, and migration of canine non-tonsillar squamous cell carcinoma. J Vet Med Sci. 2023;85(10):1068–73.
Si Y, et al. Multidimensional imaging provides evidence for downregulation of T-cell effector function by MDSC in human cancer tissue. Sci Immunol. 2019;4:eaaw9159.
Simčič P, et al. Electrochemotherapy in treatment of canine oral non-tonsillar squamous cell carcinoma. Vet Comp Oncol. 2020;18:428–32.
Singhal S, et al. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell. 2016;30:120–35.
Thaiwong T, Sledge DG, Collins-Webb A, Kiupel M. Immunohistochemical characterization of canine oral papillary squamous cell carcinoma. Vet Pathol. 2018 Mar;55(2):224–232. doi:10.1177/0300985817741732
Todoroff RJ, Brodey RS. Oral and pharyngeal neoplasia in the dog: a retrospective survey of 361 cases. J Am Vet Med Assoc. 1979;175:567–71.
Wang Z, et al. CD62Ldim neutrophils specifically migrate to the lung and participate in the formation of the pre-metastatic niche of breast cancer. Front Oncol. 2020;10:540484.
Wei W, Hu-Jie C. Association of the infiltration of tumor-association macrophages, expression of Smad7 protein and prognosis in oral squamous cell carcinoma. Arch Oral Biol. 2022;95:22–9.
Yan M, et al. Roles of tumor-associated neutrophils in tumor metastasis and its clinical application. Front Cell Dev Biol. 2022;10:938289.
Zhao JJ, et al. The prognostic value of tumor-infiltrating neutrophils in gastric adenocarcinoma after resection. PLoS One. 2012;7:e33655.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Brazilian Journal of Veterinary Pathology

This work is licensed under a Creative Commons Attribution 4.0 International License.

